bottle-point adsorption test|Ion : Chinese The adsorption of carbon tetrachloride has been studied in a batch adsorption test using pulverized GAC. In the tests, a small amount of CCl4 was added to bottles containing water .
webWatch Namorada Cavalgando porn videos for free, here on Pornhub.com. Discover the growing collection of high quality Most Relevant XXX movies and clips. No other sex tube .
{plog:ftitle_list}
Узнай больше. 8 (3452) 690-750. Выберите тему вашего вопроса: HTML-комментарий Образовательные услуги на возмездной основе Вопросы перевода из других вузов .

To perform experiments relevant to natural water, it can be advantageous to use a medium with the same major ionic composition as the natural water being studied, but without natural constituents, such as trace components, colloids and organic substances. In this study, the freshwater characterization of a . See moreThe raw zeolitic material used in this study was the natural clinoptilolite (NC) that was taken from a sedimentary deposit near of the Tehuacan region in the state of Puebla, Mexico. This . See moreAlthough batch laboratory adsorption studies provide useful data and parameters on the application of adsorbents for hardness removal, the data obtained from batch adsorptive system are not applicable to continuous adsorptive system, thus continuous sorption studies are needed. Fixed-bed column experiments were also necessary to . See more Highlights. •. Demonstrated the impact of the bottle-point method upon sorption isotherms. •. Used the Langmuir Vageler model to standardize sorption equilibrium data. •. .
and simple tests that yield information about the number of high-energy adsorption sites on carbon: a parameter that is theorized to be more closely indicative of the ability of a particular .
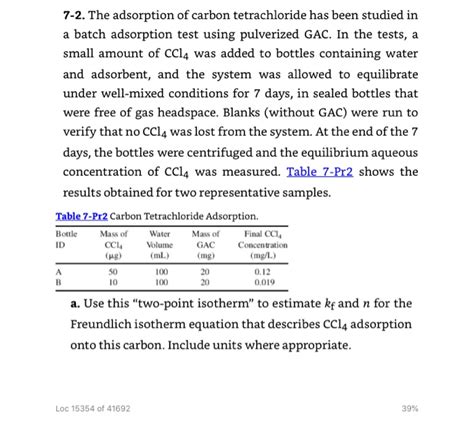
The adsorption of carbon tetrachloride has been studied in a batch adsorption test using pulverized GAC. In the tests, a small amount of CCl4 was added to bottles containing water .The adsorption of carbon tetrachloride has been studied in a batch adsorption test using pulverized GAC. In the tests, a small amount of CCl4 was added to bottles containing water .
Adsorption of heavy metals (Cr, Cd, Pb, Ni, and Cu) onto Activated Teff Straw (ATS) has been studied using batch-adsorption techniques. This study was carried out to .Bottle-Point Method 1: Constant Resin Mass and Variable Concentration of Solute In the published ion exchange literature as mentioned above, the bottle-point method involving . The batch adsorption process is one of the most practical approaches used to adsorb pollutants from the liquid solution for the purification of water. This chapter presents .This Test Guideline is aimed at estimating the adsorption/desorption behaviour of a chemical on different soil types.
Test No. 106: Adsorption
Bottle point adsorption kinetic experiments with the selected resins and resin beads were conducted in 1 L polypropylene opaque bottles (VWR, West Chester, PA) at 150 . Catalyst adsorption isotherm tests BTEX adsorption isotherms were tested using the method of constant concentration bottle point (Millar et al. 2015). Different quantities of prepared Co-ZSM-5 . Adsorption isotherms. Bottle-point. adsorption tests were used in this. study. Each HAA solution was pre- . the bottle-point isotherm test is much easier to conduct in. the absence of biological .
Fo si pli it , e a te this ethod Co sta t Mass bottle-point test. The second bottle-point variant involves the addition of various masses of sorbent material to vessels which hold constant volumes of solution comprising a constant . Bottle-point adsorption tests were used to obtain the adsorption isotherm s of five different PAMs ( i.e. , 1.5 K, 10 K, 0.6–1 M, 5–6 M, and 18 M PAMs) [23] . The kaolinite suspension and PAM .the reaction end point is indicated by a change in color of the indicator from red to blue. INSTRUCTIONS • Remove the cap and fill the Demineralizer Bottle with tap water. • Replace the cap and shake for at least 2 minutes. The demineralized water is now ready. • Determine the conductivity of your sample using the Adsorption isotherms for argon at 87 K in the ink-bottle pore and the corresponding unit pores. Solid symbols represent adsorption branches and open symbols desorption branches.
approach, the test duration rarely exceeds 96 hours, i.e. leaching behaviour is monitored for a relatively short period. In some cases, test durations are kept as low as 24 hours [2]. Also, it almost became a standard procedure to conduct the test on finely ground ore (usually .
Plastic packaging systems for pharmaceutical use include, among others, ampoules, bags, blisters, bottles, cartridges, inhalers, prefillable syringes, pouches, vials and their associated materials and secondary components like labels, inks and printing overpouches (USP 41 <661.1>, 2017).Medical devices could range from simple devices to test equipment .
For each bottle point of the adsorption isotherm curve, the test bottles were triplicated for quality control and assurance. Additionally, a set of control systems without kaolinite addition were prepared, to check the PAM loss in the aqueous phase by physicochemical processes other than adsorption on kaolinite; to generate eight points on a .This Test Guideline is aimed at estimating the adsorption/desorption behaviour of a chemical on different soil types. The goal is to obtain a sorption value which can be used to predict partitioning under a variety of environmental conditions to this end, equilibrium adsorption coefficients for a chemical on various soils are determined as a function of soil characteristics (organic carbon .
water vapor permeability test procedure distributor
The adsorption kinetic study showed that the adsorption behavior of MB followed pseudo-second order kinetics (R 2 = 1) and Langmuir isotherm adsorption model (R 2 = 0.947), and chemisorption was . For simplicity, we can term this method “constant mass” bottle-point test. The second bottle-point variant involves the addition of various masses of sorbent material to vessels which hold constant volumes of solution comprising a constant concentration of solute . For adsorption studies, the isotherm is valid for a certain system of .Experimental Data. The following data are based upon measurements described in J.H. Potgieter, Journal of Chemical Education, 68, 349 (1991). For each trial, a given amount of activated carbon (indicated below in milligrams) was measured into a small flask. 100 mL of a 25 mg/L methylene blue stock solution was then added to each flask.
water vapor permeability testing equipment distributor
The N2 adsorption isotherm of meso-ZSM-5, as expected to have a much larger adsorption amount than that of ZSM-5, has a steep uptake below P/P0 = 0.02 and a hysteresis loop above P/P0 = 0.6. Thus, the co-presence of micropores and mesopores is suggested by the N2 adsorption isotherm.(Tao, Y. S. etal.; J. Am. Chem. Soc. 2003, 125, 6044-6045) in certain titrations to determinate the end point using an adsorbent as indicator (Example: Flouroscein). Procedure of adsorption: 500 ml of 0.5N oxalic acid solution is prepared. Five well cleaned, dried, reaction bottles (250 ml) are taken and are labeled. The batch adsorption process is one of the most practical approaches used to adsorb pollutants from the liquid solution for the purification of water. This chapter presents literature of different adsorption types with its characteristics, including an overview of different factors influencing the batch process. . The pH at which the surface .
In order to further investigation for the FMFN structure, the N 2 adsorption and desorption isotherm test at 77 K was performed. As can be seen, the isotherm of FMFN showed close to Type IV with very obvious hysteresis loop in the relative pressure range of 0.4–1.0 and capillary condensation suggesting the co-existence of mesopores and .The N 2 adsorption method is mainly to measure the specific area and the characteristics of pore size of mesopores and macropores (3–109.8 nm). The CO 2 adsorption method is mainly to measure the specific area and the pore volume of the micropores (0.3–1.5 nm). This test was carried out in the Beijing Center for Physical and Chemical Analysis.1.1 This test method covers the determination of the relative activation level of unused or reactivated carbons by adsorption of iodine from aqueous solution. The amount of iodine absorbed (in milligrams) by1gofcarbon using test conditions listed herein is called the iodine number. 1.2 This standard does not purport to address all of thePost test 25 Learn with flashcards, games, and more — for free. . All containers, bottles, and labels from the substance. In a patient who is experiencing tachycardia, hypertension, nausea, and tremors, you should most suspect: . binding poisons through adsorption, thus preventing poisons from being absorbed by the body.
A literature review of the five main theories describing the interaction mechanisms in the bitumen/aggregate system was conducted: theory of weak boundary layers, mechanical theory, electrostatic theory, chemical bonding theory, and thermodynamic theory (adsorption theory). The adhesion assessment methods in the bitumen/aggregate system are described, . For simplicity, we can term this method “constant mass” bottle-point test. The second bottle-point variant involves the addition of various masses of sorbent material to vessels which hold constant volumes of solution comprising a constant concentration of solute [20], [21], [22]. This method we call the “constant concentration” bottle .
Solved The adsorption of carbon tetrachloride has been
Isotherms were developed from bottle-point adsorption experiments for the four different particle size fractions. One-liter bottles (HDPE Nalgene) were filled with a test solution and pre-weighed masses of GFH. Bottles were agitated on an orbital shaker table at a reciprocating speed of 100 rpm for 18 days.Compatibility test: This test is performed to check the compatibility of the rubber closures with various types of the substances, since it is necessary to ensure that there is no interaction between the contents of the bottle and the closure. 6.Light absorption Filter solution A through membrane filter. Measure the light absorbance of filtrate . Batch adsorption test. Batch adsorption tests for Tl(I) removal by TMS-SMSs was performed using the traditional bottle-point technique in 50 mL polypropylene (PP) bottles and consist of the effect of solution pH and adsorbent dose on adsorption, adsorption isotherm tests, adsorption kinetics, and thermodynamic studies. . To examine the mechanism of the adsorption hysteresis in ink-bottle pores, we measured the temperature dependence of the adsorption–desorption isotherms of argon, oxygen, and carbon dioxide onto .
The adsorption of Pb(II) on silica gel synthesized from chemical glass bottle waste has been studied. The effect of independent variables (adsorbent dose, initial concentration of Pb(II), contact time, and pH) on the Pb(II) removal from water was evaluated and optimized using the Response Surface Methodology (RSM).
Solved 7
Resultado da Os melhores quadrinhos pornô gay da internet. Confira também vídeos eróticos polêmicos com diversos temas: interracial, pênis gays, famosos, .
bottle-point adsorption test|Ion